Point of patient impact
Mastering the Point of Patient Impact
Vertically integrated manufacturing for medical device OEMs, delivering speed, precision, and confidence from concept through commercialization.
At Cadence, manufacturing excellence is measured at the Point of Patient Impact — the place where design intent becomes clinical performance. By focusing on patient-critical features within a unified manufacturing and quality system, we help OEMs reduce risk, accelerate development, and deliver reliable, high-performance devices to patients.



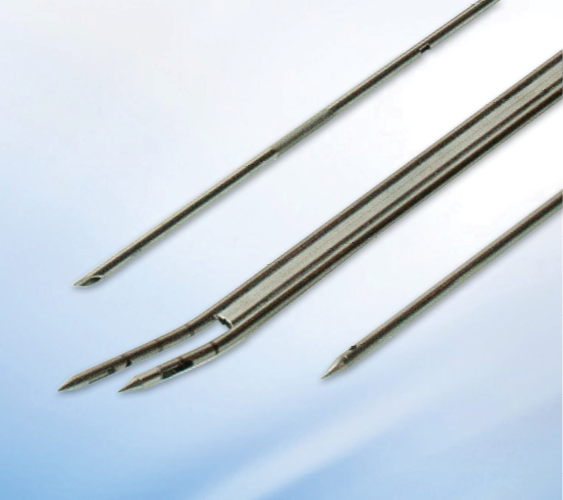
Where Designs Become Life-Changing Devices
See how Cadence turns complex engineering into dependable, real-world performance for the patients who rely on your device’s performance.
What Is the Point of Patient Impact?
The Point of Patient Impact is the critical interface where a medical device directly contacts the patient (needle/blade) and the manufacturing precision influences clinical performance, patient safety, and reliability in use.
This is where:
- Tolerances define function
- Surface finish affects tissue interaction
- Geometry determines dose delivery
- Cleanliness and consistency protect patient safety
When failure is not an option, Cadence excels in mastering the manufacturing details that define those unique patient outcomes.
Why Medical Device OEMs Choose Cadence
Medical device development is increasingly complex, regulated, and time-sensitive. Fragmented supply chains introduce risk, delay, and diluted accountability. Cadence eliminates these challenges through a vertically integrated model that unifies the entire product lifecycle within one accountable partner.
Reduced Risk at the Most Critical Point
By owning the Point of Patient Impact, Cadence minimizes variability, focuses on quality, and the critical features that matter most.
Faster Time to Market
Early engineering engagement and manufacturing involvement eliminates confusion during product launches, transfers / handoffs, and can prevent late-stage redesigns.
Superior Performance and Reliability
Tight control over materials, processes, tolerances, and cleanroom assembly ensures consistent execution of patient-critical features as you scale.
Simplified Supply Chain
One partner. One quality system. Accountability.
Vertically Integrated from Concept to Commercialization
Cadence’s vertically integrated manufacturing model consolidates design support, component manufacturing, finished device assembly, validation, and commercialization within one organization.
OUR CORE CAPABILITIES INCLUDE:
End-to-End Manufacturing Solutions
Achieve unparalleled precision and consistency with our state-of-the-art manufacturing capabilities:
- Engineering: Strong structure, discipline, and technical approach, from concept through commercialization, backed by a team of highly skilled engineers, bring products to reality that help improve patient outcomes.
- Our vertically integrated capabilities are a key driver in our end-to-end solutions.
Cadence is the leading CDMO for:
- Precision machining, metal stamping, deep draw, and MIM
- Plastics molding, overmolding, and insert molding
- Laser processing, sharpening, cutting, welding, and marking
- ISO Class 7 & 8 cleanroom assembly, packaging, and labeling
- Sterilization management and validated production processes
This integration reduces risk, shortens timelines, and delivers results.
Engineering & NPI
Cadence engages early in the product lifecycle to ensure devices are optimized for manufacturability, compliance, and patient performance from the start. We are your trusted partner from concept through commercialization.
Our team provides:
- Program Management: Cadence’s NPI process is designed to bring your innovative medical devices to market with unparalleled speed and precision, and our Program Management services are the cornerstone of successful medical device manufacturing projects.
- Design for Manufacturability (DFM): Cadence specializes in manufacturing custom products to meet the unique challenges of our OEM customers. From complex components and sub-assemblies to finished devices, we are your “single source” contract manufacturing partner, leveraging advanced technologies for difficult-to-manufacture products.
- Design for Assembly (DFA): We provide DFA to help make products easy and efficient to assemble as a part of our value-add engineering services, providing optimized assembly design, reduced costs, improved efficiencies, and long-term program success.
- Validation: As a leading Medical Device Contract Manufacturing Organization (CMO), we ensure every piece of equipment and process is meticulously validated to guarantee consistent, high-quality production. Our team prioritizes the highest standards of reliability and compliance through our comprehensive validation services, encompassing Installation Qualification (IQ), Operational Qualification (OQ), Performance Qualification (PQ), and Process Validation.
Quality & Regulatory
Quality and regulatory compliance are foundational at Cadence. Our unified Quality Management System is certified to ISO 13485:2016 and compliant with FDA cGMP (21 CFR 820), governing all sites and capabilities under one disciplined framework.
We apply:
- Integrated risk management aligned to ISO 14971
- IQ/OQ/PQ validation with centralized corporate oversight
- GAMP-5 aligned equipment and automation lifecycle management
- Full traceability and audit readiness across the product lifecycle
As the listed manufacturer for numerous Class II and III medical devices, Cadence serves as an extension of our customers’ quality systems, delivering compliant, validated, and inspection-ready products from clinical builds through commercial production.
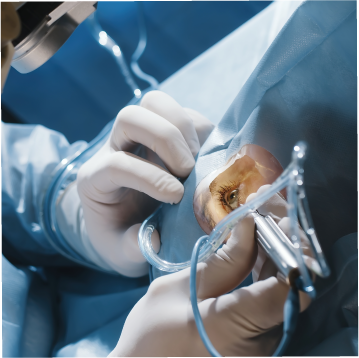
Confidence at the Point of Patient Impact
Cadence doesn’t just manufacture devices — we set the standard for the features that define performance, safety, and patient outcomes. By mastering the Point of Patient Impact through vertical integration and validated execution, Cadence empowers medical device OEMs to bring life-changing technologies to market faster, safer, and with confidence.

Case Study
Innovating at the Point of Patient Impact
A leading medical device OEM partnered with Cadence to solve a critical manufacturing challenge: scale production of an existing needle-based device while reducing piece price and minimizing handling at a patient-critical feature. Because the needle directly impacts clinical performance, precision, consistency, and quality at scale were essential.
Using our vertically integrated manufacturing model, Cadence evaluated the product design, manufacturing process, and quality requirements as a single, unified system. The team proposed a novel continuous strip-based process to remove burrs in-line, which significantly reduced the need for manual handling and improved consistency and throughput.
The next step – Cadence engineers designed, built, and validated a custom prototype to prove the concept. By integrating process development, tooling, automation, and quality engineering under one roof, we rapidly transitioned innovation into a scalable, production-ready solution.
The result exceeded the OEM’s performance expectations, enabled high-volume manufacturing with tighter process control, and delivered a higher-quality device at the Point of Patient Impact—ultimately improving outcomes for patients worldwide.
How Can We Help You?
"*" indicates required fields
